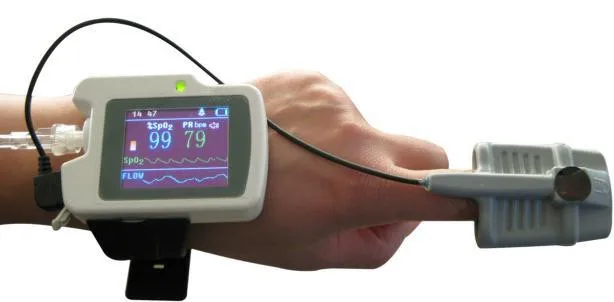
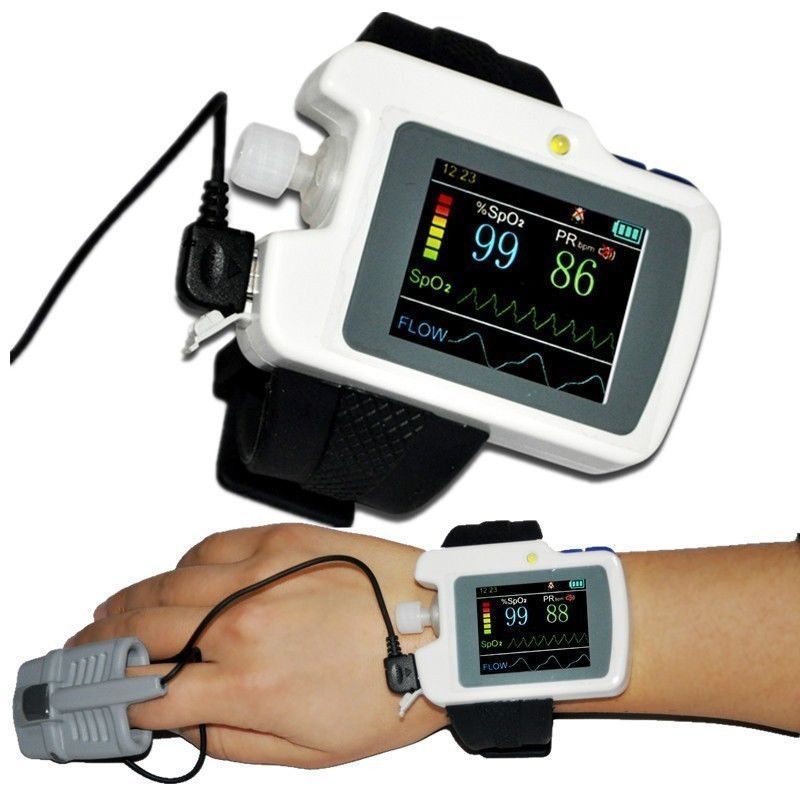
Description
Apnea Screen Meter, Wrist Watch Respiration Sleep Monitor, PC Software

Instructions The RS01 device is applied for the suffers with such diseases as OSAHS(obstructive sleep apnea-hypopnea syndrome), COPD(chronic obstructive pulmonary diseases) and vascular diseases, also for the persons over 60 years .It can be used in hospital or at home.
Main Features
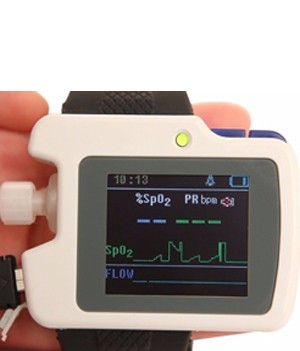
- 1.8″ OLED display screen
- Convenient operation
- Wrist-design,tiny and light
- Multiple functions in one device,supplying more vital information
- SpO2 and pulse rate(hereinafter referred to as “PR”) value display
- Pulse sound and indicator bar
- Pulse and nose flow waveform display
- Such alarms as low power, finger out and exceeding limits can be set up
- The degree for screen back-light is adjustable
- Real-time clock
- Auto-power on/off
- Multi-case record
- Continuous 20 hours data record
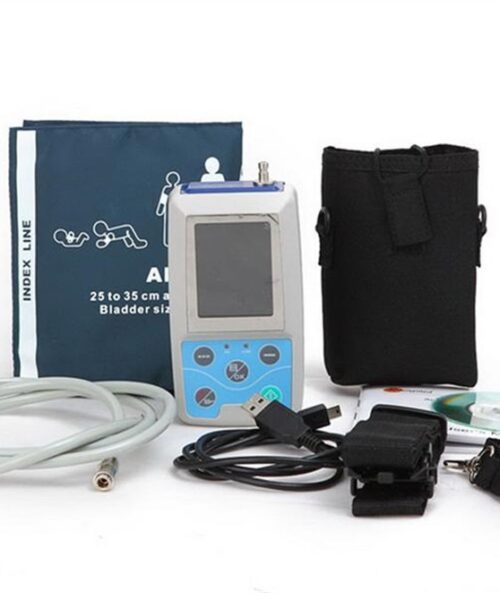
- Uploading Data via USB port
- Powerful PC analysis software
Performance parameters
(1) Nose air flow measurement:
- Measurement range:0rpm~40rpm
- Resolution:1rpm
- Accuracy:±2rpm
(2) SpO2 measurement:
- Measurement range:0%~100%
- Resolution:1%
- Accuracy: 70%~100% ,±2% ;<70% unspecified
(3) Pulse rate measurement:
- Measurement range:30bpm~250bpm
- Resolution:1bpm
- Accuracy: ±2 bpm and ±2%(selecting larger)
(4) Measurement Performance in Weak Filling Condition
- SpO2 and pulse rate can be shown correctly when pulse-filling ratio is 0.4%. SpO2 error is ±4%, pulse rate error is ±2 bpm or ±2% (selecting larger).
(5)Resistance to surrounding light:
- The deviation between the value measured in the condition of man-made light or indoor natural light and that of darkroom is less than ±1%.
Specification
Power Supply : ONE 3.7V Lithium Battery
Display : 1.8″ color OLED
Dimension:69mm(L)× 50mm(W)×17.3mm (H)
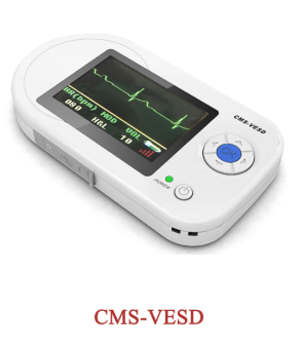
Respiration Sleep Monitor Spo2,Pulse Rate,Nose Air Flow Measure






FDA K Number
CMS50C Pulse Oximeter
Premarket Submission Number(510K): K073454
Listing Number: D045684
CMS50D/L/DL Pulse Oximeter
Premarket Submission Number(510K): K082641
Listing Number: D064765
Sonline A/B,Baby Sound A/BPocket Fetal Doppler
Premarket Submission Number(510K): K082480
Listing Number: D072247
CMS50E,CMS50F,CMS60C,CMS60D , Pulse Oximeter
Premarket Submission Number(510K): K090671Listing Number:D078664,
ECG80A ECG machine
Premarket Submission Number(510K): K090936
Listing Number: D081157
| Weight | 0.75 lbs |
|---|---|
| Dimensions | 27 × 15 × 7 in |
| Brand Name: | CONTECMED |
| Item Type: | Blood Pressure |
| Application: | Finger |
| Model Number: | RS01 |
| Commodity Quality Certification: | CE |
Only logged in customers who have purchased this product may leave a review.
General Inquiries
There are no inquiries yet.



























There are no reviews yet.